When you pick up a prescription, you might see two options: the familiar brand name you’ve seen on TV, or a much cheaper generic version with a plain label. Many people wonder - is the generic just as good? Or are you saving money at the cost of your health?
The short answer? For most people, generic drugs work exactly the same as brand-name ones. The science behind this isn’t complicated, but the confusion around it is. Let’s cut through the noise.
What Makes a Generic Drug ‘Generic’?
A generic drug isn’t a copycat or a knockoff. It’s the exact same medicine, made to meet the same strict standards as the original. The active ingredient - the part that actually treats your condition - is identical. So if your brand-name pill has 10 milligrams of atorvastatin, so does the generic. Same strength. Same shape. Same way it’s taken - whether it’s swallowed, injected, or inhaled.
The only differences are in the inactive ingredients: things like fillers, dyes, or coatings. These don’t affect how the drug works. They’re there to help with manufacturing, shelf life, or making the pill easier to swallow. Sometimes, these minor changes can cause slight differences in how fast the drug gets into your bloodstream - but not enough to matter for most people.
The Bioequivalence Rule: How the FDA Makes Sure Generics Work
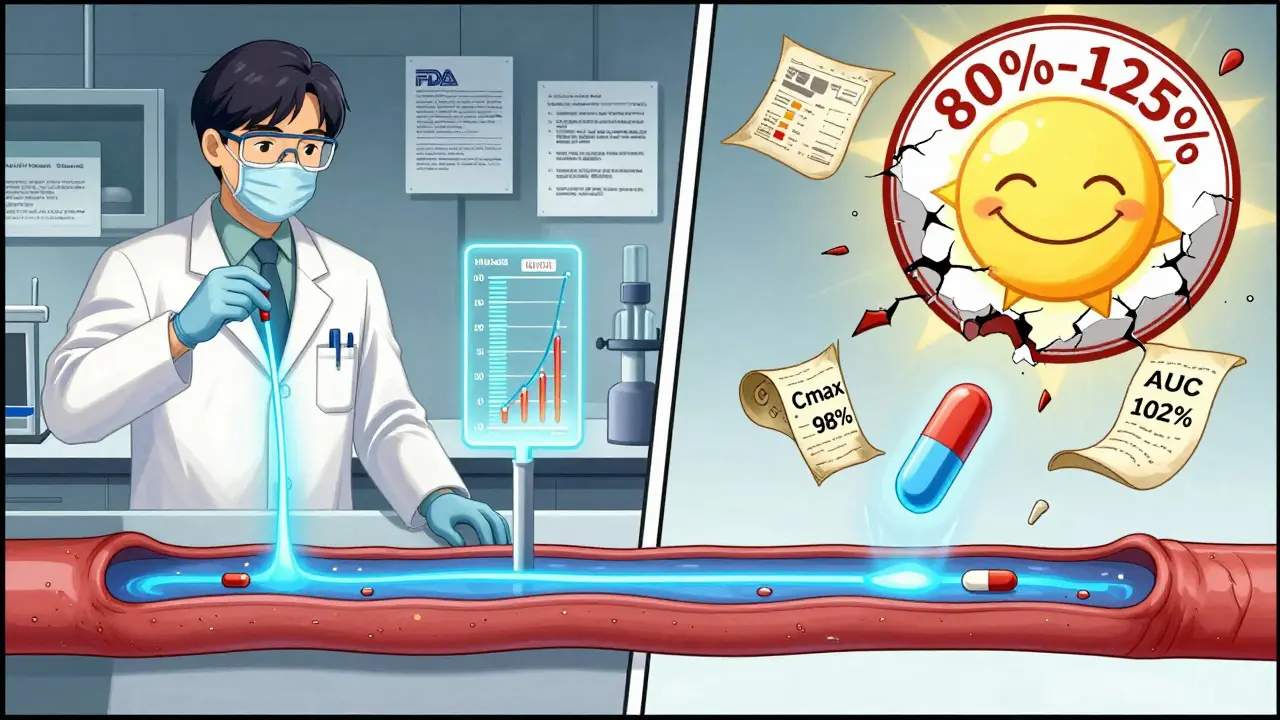
The U.S. Food and Drug Administration (FDA) doesn’t let just any company slap a generic label on a pill. To get approved, the maker must prove bioequivalence. That means the generic drug must enter your bloodstream at the same rate and to the same extent as the brand-name version.
Here’s how they test it: In clinical trials with 24 to 36 healthy volunteers, researchers measure two key things - how high the drug peaks in your blood (Cmax) and how much of it stays in your system over time (AUC). The generic’s numbers must fall within 80% to 125% of the brand’s. That’s not a wide range. It’s tight. In fact, studies show the average difference in absorption between generics and brand names is just 3.5%.
And here’s the myth that needs busting: this 80%-125% range does NOT mean the generic contains only 80% of the active ingredient. That’s a common misunderstanding. The active ingredient is 100% the same. The range applies only to how your body absorbs it - and even then, 98% of studies show absorption differences under 10%.
Cost Savings That Actually Matter
Let’s talk numbers. A 30-day supply of brand-name Lipitor (atorvastatin) used to cost over $300. Today, the generic? Often free with coupons. Plavix? Around $450 for the brand. The generic? $0. That’s not a discount. That’s a revolution.
In 2023, generic drugs saved the U.S. healthcare system $373 billion. That’s billions of dollars that didn’t go to drug companies - and instead stayed in patients’ pockets, or helped keep insurance premiums lower. Generics make up 90% of all prescriptions filled in the U.S., but only 23% of total drug spending. That’s the power of competition.
And it’s not just Americans saving. Globally, the generic drug market hit $462 billion in 2023, growing faster than the brand-name market. In New Zealand, where I live, the government actively encourages generic substitution to keep public health costs manageable. It’s not just smart - it’s necessary.
When Generics Might Need Extra Care
There’s one big exception: drugs with a narrow therapeutic index (NTI). These are medications where even tiny changes in blood levels can cause serious problems - either the drug stops working, or it becomes toxic.
Examples include warfarin (a blood thinner), levothyroxine (for thyroid issues), phenytoin (for seizures), and lithium (for bipolar disorder). For these, switching from brand to generic - or even between two different generics - can sometimes require extra monitoring.
That doesn’t mean you can’t use generics. It means you need to be watched more closely. For example, if you’re on warfarin and switch to a generic version, your doctor might check your INR levels within 7 to 14 days to make sure your blood is still clotting at the right rate. Most patients do just fine. But the extra step matters.
Studies show that even with NTI drugs, outcomes are often the same or better with generics. One large Austrian study of over a million patients found that for 10 out of 17 drug classes, generic versions were linked to fewer deaths than brand names. That’s not a fluke. It’s data.
Why Do Some People Still Doubt Generics?
If the science is so clear, why do 43% of patients believe generics are less effective? And why do nearly 3 in 10 refuse to take them when offered?
Part of it is marketing. Brand-name companies spend billions on ads that make their drugs feel like the only safe choice. They don’t say it outright, but the message is: “Our version is better.”
Another part is personal experience. Some people report feeling different after switching - headaches, fatigue, or a sense that the medicine isn’t working as well. Often, these are placebo effects. Or, they’re caused by switching between two different generics, each with slightly different inactive ingredients. One person might switch from Brand A to Generic B, then later to Generic C, and blame the generic when the real issue is constant switching.
On Reddit, one thread with over 400 comments found that 67% of people saw no difference between brand and generic. But the 28% who did notice changes? They were loud. And their stories stick.

What You Can Do
If your doctor prescribes a brand-name drug, ask: “Is there a generic?” If you’re already on a generic and feel fine - don’t switch unless your pharmacist or doctor recommends it.
For most medications - statins, blood pressure pills, antibiotics, antidepressants - generics are the smart, safe, and affordable choice. The FDA says they have the same risks and benefits. That’s not a marketing slogan. It’s the law.
For NTI drugs, don’t panic. Just be aware. Talk to your pharmacist. Ask if your generic is AB-rated (the highest equivalence rating). Check the FDA’s Orange Book if you want to dig deeper. And if you switch, schedule a follow-up test if your doctor recommends it.
There’s no reason to pay more unless you have a proven reason to. And even then, it’s not about the drug being inferior - it’s about managing your body’s response.
What’s Next for Generic Drugs?
The FDA is speeding up approvals for complex generics - things like inhalers, topical creams, and injectables - with over 247 approved in 2023 alone. New technology is making manufacturing even more precise. A 2023 MIT study showed future generics for warfarin could reduce absorption variation to under 2% - nearly invisible.
But there’s a warning sign: drug shortages. In 2023, there were 312 shortages of generic drugs, mostly sterile injectables. That’s up 17% from the year before. Supply chains are fragile. That’s why having multiple generic makers for the same drug matters.
And soon, biosimilars - the next generation of generics for complex biologic drugs like Humira or Enbrel - will become more common. These won’t be exact copies, but they’ll be close enough to save patients tens of thousands a year.
Generics aren’t the future. They’re the present. And for most people, they’re the best choice - safe, effective, and affordable.

So we’re telling people to trust a system that lets the same company that made the brand-name drug also make the generic with a different label and still calls it ‘bioequivalent’? Sure. And I believe the moon landing was real too. Just saying.
People don’t care about the 3.5% absorption difference. They care that their anxiety got worse after switching. Or their headaches started. Or their sleep vanished. Science doesn’t measure how you feel. It measures what’s in the blood. Big difference.
I’m not anti-generic. I’m pro-not-ignoring-real-people-who-say-things-don’t-work-the-same. The FDA’s 80-125% range is basically saying ‘close enough’ and then acting like that’s a guarantee. It’s not. It’s a statistical loophole.
And don’t get me started on the fact that generics are often made in factories with less oversight. Yeah yeah ‘same standards’ - but standards are enforced differently when the regulator’s budget got cut by 40% last year.
So yeah. Save your money. But don’t pretend your body won’t notice the difference. Some of us aren’t lab rats in a 36-person trial.
Also - why do they always say ‘for most people’? Who are the ‘most people’? The ones who don’t talk on Reddit? The ones who don’t get sick? The ones who don’t care enough to complain? I’m tired of being part of the statistical majority that doesn’t matter.